Newsroom
View news releases and announcements distributed by EZ Newswire, the trusted source for business news.
Advocates React to Georgia Ban Bill That Would Turn Law-Abiding Consumers Into Felons
Legislation introduced by Representative Rick Townsend would impose an outright ban on natural kratom leaf products in Georgia — a sweeping move that directly contradicts the stated positions of the U.S. Department of Health and Human Services and the U.S. Food and Drug Administration. Townsend’s bill ignores the Trump Administration’s clear focus on dangerously formulated synthetic threats to consumer safety.
Townsend’s bill (HB 968) makes two terrible mistakes: (1) the bill fully bans both natural leaf kratom products (mitragynine) and dangerously addictive synthetic and chemically formulated products called 7-hydroxymitragynine (7-OH) as Schedule I substances; and (2) and repeals Georgia’s existing Kratom Consumer Protection Act. The Townsend bill will instantly convert hundreds of thousands of law-abiding Georgia consumers into felons and expose them to extended jail terms, despite their use of products that are currently lawful and safely formulated under Georgia law.
Federal leaders have been clear: the real danger to consumers is highly concentrated synthetic, chemically manipulated 7-OH knock-offs — not responsibly manufactured natural kratom leaf products.
At a July 29, 2025, media event addressing the scheduling of 7-OH, FDA Commissioner Marky Makary underscored that enforcement is aimed at synthetic and chemically manipulated products and not natural kratom leaf products consumed responsibly by adults.
Likewise, HHS Secretary Robert F. Kennedy, Jr., has aligned federal health policy toward targeting synthetics and illicit manufacturing practices — precisely the opposite of Rep. Townsend’s approach, which sweeps safe, regulated products into his felony dragnet.
“Rep. Townsend’s bill not only fails to protect consumers, it puts kids at high risk,” said Mac Haddow, senior fellow on Public Policy for the American Kratom Association. “He has personally criticized Georgia’s existing kratom law for weak enforcement — yet he has repeatedly refused to support the very enforcement authorities the American Kratom Association has advocated to target bad actors and dangerous synthetics. This bill abandons smart regulation in favor of mass criminalization.”
The consequences are immediate and severe. By repealing Georgia’s Kratom Consumer Protection Act and scheduling naturally occurring constituents found in the kratom leaf, the Townsend bill would:
- Criminalize responsible adult consumers in Georgia overnight;
- Eliminate age limits, labeling, testing, and manufacturing standards that currently protect Georgians that is in the Georgia Kratom Consumer Protection Act;
- Divert law enforcement away from dangerous synthetics and toward kratom consumers who benefit from properly regulated kratom products;
- Stand in direct conflict with the FDA’s and HHS’s stated priorities; and
- Create a black market for dangerously adulterated kratom products that puts Georgia kratom consumers — and kids — at risk for products laced with illicit drugs like fentanyl.
Georgia already has a solution that works: regulation.
Safely formulated kratom products — tested, labeled, and sold with age restrictions. Today, these safely formulated natural kratom leaf products are consumed by hundreds of thousands of Georgians without incident. Rep. Townsend’s bill tears down those safeguards and replaces them with a prohibition that federal health leaders have explicitly rejected.
Bottom line: Rep. Townsend is not protecting consumers. He is ignoring the chief health authorities in the Trump Administration who protect the health and safety of consumers, undermining proven state safeguards, repeats an outdated and discredited anti-kratom narrative pushed by ambulance chasing trial lawyers, and is pushing a prohibition that unfairly punishes responsible Georgia natural kratom leaf consumers.
For what purpose: To provide Rep. Townsend another few minutes in front of a camera while he puts responsible Georgia kratom consumers in front of a judge.
About American Kratom Association (AKA)
Media Contact
Mac Haddow
Senior Fellow on Public Policy
mhaddow@americankratom.org



TORAD Announces Breakthrough Spool Compressor Performance to Address Global Warming
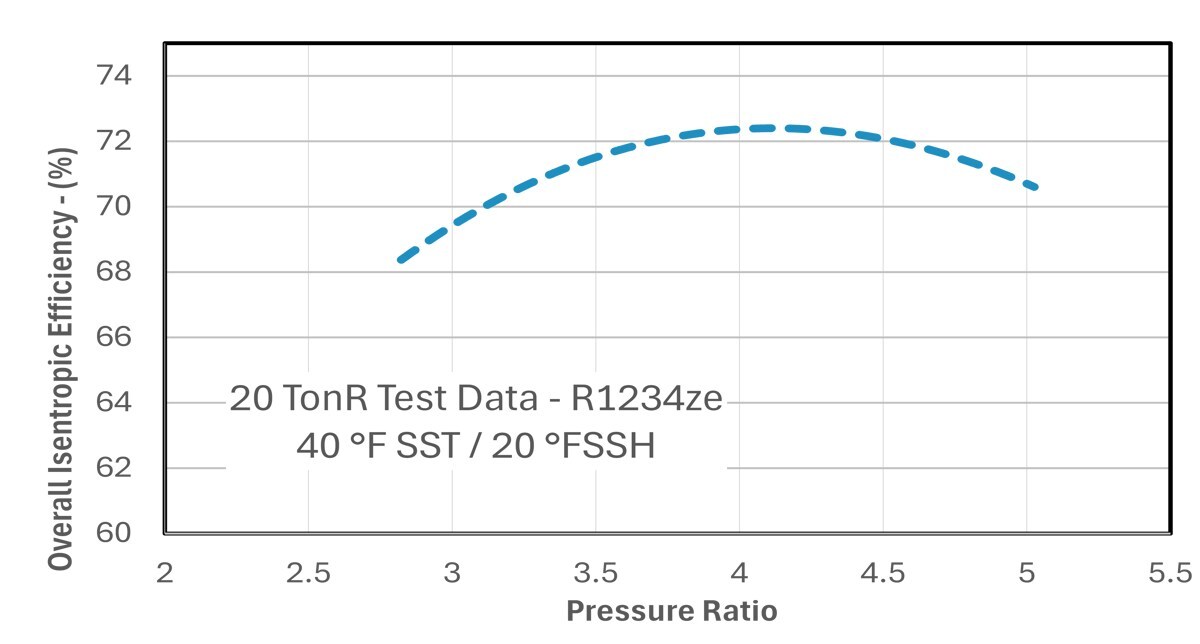
TORAD Inc., a leader in innovative compressor technology, today announced that its patented spool compressor has achieved breakthrough performance of 72.5% peak overall isentropic efficiency running on Solstice ZE (R-1234ze), a zero-global warming potential (GWP) refrigerant. The 20 TonR compressor was tested at the ARI standard condition of 45°F/130°F, achieving an Energy Efficiency Ratio (EER) of 12.2 and a Coefficient Performance (COP) of 3.58.
Joe Orosz, TORAD president and COO said, "This achievement creates a viable path to commercial HVAC adoption of environmentally sustainable zero-GWP systems without the cost typically associated with next-generation refrigerants." Orosz added, "The company's spool compressor technology enables advanced chiller designs improving system efficiency and addressing the current industry trade-off between cost and environmental impact."
Greg Kemp, TORAD CEO and founder stated, "The successful combination of the spool compressor and Solstice ZE creates a cost-effective path to meet challenging global regulatory demands for sustainable cooling solutions and dramatically lowering greenhouse gas emissions."
Kemp added, "We would like to thank Johnson Controls International, Solstice, Shrieve, Syensqo, GGB, CLAYENS and Force Technology for their valuable engineering support in this achievement. We look forward to working with industry partners to bring this technology into commercial production and accelerate the global transition to sustainable cooling solutions."
About TORAD
TORAD Inc. is an Atlanta-based leader in the development of innovative, high-efficiency compressor technologies for the HVAC and refrigeration industries. Dedicated to sustainability and performance, TORAD is committed to delivering climate tech solutions that meet the evolving environmental and economic demands of the global market. For more information, visit www.toradengineering.com.
Media Contact
Media Relations
info@toradengineering.com


Mars Materials Accepted into Shell GameChanger Program to Accelerate Alternative Acrylonitrile Pathway
Mars Materials ("MM" or the Company), a company working to store captured carbon dioxide into everyday products, today announced its selection into Shell GameChanger and successful advancement past the program's first stage gate. The program aims to validate an alternative, bio-based feedstock for MM's proprietary acrylonitrile production pathway.
MM's acrylonitrile is a key chemical building block for carbon fiber, water production polymers, durable plastics, textiles and more, is hydrogen cyanide-free and has been validated by global industry. Produced at MM's pilot plant, Cassini, the team has successfully scaled its novel process from gram-scale to kilogram-scale production of acrylonitrile.
The Shell GameChanger test program aims to validate bio-based sugar feedstock as an alternate raw material to CO2 for the MM's acrylonitrile pathway.
"We're beyond excited to participate in Shell GameChanger, a program that exemplifies how startups and corporations can innovate together to unlock commercial opportunity," said Aaron Fitzgerald, CEO and co-founder of Mars Materials. "Shell's support has already accelerated our development by an estimated three years. Shell GameChanger will help expand our choice of raw material with a bio-based sugar option, giving us another edge over the incumbent acrylonitrile production process, which relies on a globally distributed supply chain."
In addition to technical guidance, the Shell GameChanger program will help MM with support in market discovery as well as commercial demonstration and go-to-market planning.
Having successfully passed the first stage gate of the Shell GameChanger program, MM continues to demonstrate that its partner-first approach works. The company intends to replicate its success and seeks to attract further investment, grant funding, and strategic industrial partners necessary for the next phases of its scale-up and commercialization.
About Mars Materials
Mars Materials, Inc. PBC ("MM") is a Houston, TX-based, venture- and Breakthrough Energy Fellows-backed, carbon dioxide utilization startup. MM transforms carbon into products that purify dirty water and makes materials such as carbon fiber. The company has the only hydrogen cyanide-free and carbon negative pathway to be validated by global industry. For more information, visit www.marsmaterials.tech.
Media Contact
Aaron Fitzgerald
CEO, Mars Materials
aaron@marsmaterials.tech



Puure Introduces New Approach to Cleaner Tap Water Across Europe
Puure®, a water filtration brand focused on everyday tap water, has introduced a new approach to cleaner drinking water as households across Europe increasingly reassess how they consume and filter water at home.
Across many European markets, concerns around plastic waste, bottled water consumption and the presence of chemical residues in municipal water supplies have contributed to a shift in how consumers think about water quality.
While tap water generally meets regulatory standards, growing awareness of contaminants such as PFAS, pesticides and industrial by products has led many households to seek additional filtration solutions at the point of use.
Puure’s approach centres on a compact, tap mounted filtration system designed to operate directly at the tap. Rather than relying on bottled water, plastic filter jugs or under sink installations, the system filters water instantly as it flows, allowing filtered water to be used for drinking and cooking without altering existing plumbing. Unlike traditional plastic filtration products, the Puure system is housed in a stainless steel casing.
The filtration process combines fine filtration with activated carbon, aiming to improve taste and clarity while preserving naturally occurring minerals found in tap water. By operating at the point of use, the system is designed to integrate seamlessly into daily household routines.
Public awareness around substances such as PFAS, often referred to as “forever chemicals,” has increased significantly in recent years. Alongside agricultural pesticides and other chemical residues, these contaminants have become part of a broader public conversation about water quality across Europe.
While regulatory approaches vary by country, interest in household level filtration has continued to grow. Puure positions its stainless steel tap mounted system as an alternative to both bottled water and disposable plastic filtration products.
By filtering water directly from the tap, the system is intended to reduce reliance on single use plastic bottles and refill based filter jugs, which often require frequent replacement and generate additional waste. Durability and long term use were central considerations in the system’s design.
The stainless steel construction is intended to offer a longer product lifespan compared with plastic alternatives, while replaceable internal cartridges allow filtration performance to be maintained over time without replacing the entire unit.
Installation requires no tools or permanent plumbing changes, making the system accessible to a wide range of households, including renters. Puure is currently expanding its availability across multiple European markets as demand grows for practical, technology led household water filtration solutions. More details can be found at puurefilters.com.
Media Contact
Puure Filters
collabs@puurefilters.com



Stonebriar Wealth Advisors Announces Release of New Book Series Focused on Financial Principles Across Life Stages
Stonebriar Wealth Advisors announced the release and upcoming publication of a series of books designed to explore foundational financial principles through the lens of timing, structure, and long-term decision-making. The firm confirmed that the series is intended to support its broader mission of improving financial understanding for individuals, families, and future generations.
Founded by Gary J. Preisser and Shea M. Swenson, Stonebriar Wealth Advisors operates as a Registered Investment Advisory firm that works with households, business owners, and families on long-term wealth planning. According to Preisser, the book series reflects concepts that have been discussed with clients for years and formalizes those ideas into written resources that can be revisited over time.
The first published title in the series, "The Essence of Wealth," has already been released. Preisser explains that the book was written to speak to the next generation within client families. The goal was to present financial concepts in a way that encourages clarity and purpose rather than focusing narrowly on accumulation. He explains the book as a way for parents and grandparents to introduce foundational ideas to children and grandchildren who are just beginning to think about money and responsibility.
Swenson explains that the firm views financial education as a long-term process rather than a single conversation. “Families often engage with financial decisions at different stages of life, and written resources allow those conversations to continue even when circumstances change,” he notes. According to him, the series of books can serve as a reference point that evolves in relevance as readers move through their own personal and professional timelines.
Another book, "The Differentiators of Wealth," is scheduled for release in the near term. “This book is an exploration of structural factors that influence long-term outcomes, including the role of timing, performance, diversification, tax considerations, and decision sequencing,” Swenson notes. “The material reflects recurring questions raised in client discussions, where individuals seek to understand not only what decisions to make, but why those decisions matter.”
Beyond these two books, the firm has outlined a broader pipeline of upcoming works. The series includes forthcoming books such as "The Timing of Wealth," "The Cash Flow Clock," and "The Psychology of Wealth," among others. According to Swenson, each book is designed to address a specific dimension of financial decision-making through consistent underlying principles.
A recurring theme across the series is the importance of timing. Preisser explains that while financial principles remain consistent, their application can vary widely depending on life stage, cash flow needs, and family dynamics.
“The books are not intended to replace professional guidance, but rather to encourage informed participation,” Preisser says. “Understanding basic success metrics and concepts enables individuals to engage more confidently in financial conversations, ask better questions over time, and ultimately achieve better outcomes.”
Stonebriar traces the origins of the series to years of repeated client discussions. Swenson explains that many of the ideas became ingrained through experience, as clients raised similar concerns and uncertainties across different stages of life. He explains the writing process as a way to formalize those explanations so readers can absorb the material at their own pace and return to it as their circumstances evolve.
“Time is the one asset you can’t replace,” Preisser says, pointing to an idea frequently referenced at Stonebriar. “The best time to plant a tree was years ago, but the next best time is now,” he says. “We believe that it’s never too late to begin making informed financial choices. The decisions we make every day can be the difference between missing and achieving our goals.”
As Stonebriar prepares additional releases within the series, the firm views the books as an extension of its broader educational mission. “Our goal is to help improve financial literacy for everyone, no matter their background, age, or net worth,” Swenson says. “When people understand where they are on their own financial journey, they can begin making decisions with far greater confidence and clarity.”
About Stonebriar Wealth Advisors
Stonebriar Wealth Advisors is a Registered Investment Advisory firm founded by Gary J. Preisser and Shea M. Swenson. The firm works with individuals, families, and business owners on long-term wealth planning grounded in timing, structure, and informed decision-making. Through advisory services and educational initiatives, including its new book series, Stonebriar focuses on improving financial understanding across life stages and helping clients engage more confidently in their financial journeys. For more information, visit www.stonebriarwealthadvisors.com.
Media Contact
Carter Frame
carter@stonebriarwa.com



World’s First Mercedes-Benz Branded City Launched by Binghatti in Meydan, Marking the Largest Real Estate Event in Dubai’s History
After the global success of their first collaboration, Binghatti and Mercedes-Benz have reunited to pursue a far greater vision, one that expands the boundaries of both luxury real estate and automotive innovation. Their partnership now returns with the unveiling of Mercedes-Benz Places | Binghatti City, the world’s first Mercedes-Benz branded city and the developer’s first masterplanned community, a monumental AED 30 billion development spanning more than ten million square feet in Nad Al Sheba.
The grand unveiling in Meydan delivered one of the most cinematic moments in Dubai’s real estate industry. Hosted by Hollywood actor Terry Crews, the night drew 25,000 guests the largest attendance at any Binghatti event to date, as a spectacular drone show lit up the skyline and the program unfolded around two historic reveal moments.
The first reveal was the exclusive Middle East showcase of VISION ICONIC, MercedesBenz’s newest concept creation, inspired by the golden era of 1930s automotive design and embodying the pure essence of the marque. The grand launch opened with a performance by legendary Italian tenor Andrea Bocelli, setting an unforgettable tone for the evening.
The second reveal then unveiled the masterplan community, an entirely new urban district comprised of twelve architecturally synchronized towers. A sweeping drone show illuminated the Meydan skyline, followed by a laser projection across Nad Al Sheba tracing the outline of the twelve-tower formation, before a massive fireworks finale crowned the night symbolizing the scale and ambition of the vision.
The masterplan will be delivered in three phases, each unveiling a new layer of this city scale vision. Across the towers, residences range from studios to 3-bedroom apartments, with Vision Iconic tower also featuring a collection of 4- and 5-bedroom homes. All of it is anchored by the development’s defining centerpiece, the iconic spiraling signature tower bringing to life one of the region’s most ambitious residential masterplans.
At the heart of the development lies the Grand Promenade, a vast green expanse imagined as a sanctuary of sustainable and environmental consciousness. Designed as a sequence of 12 unexpected moments and curated experiences, it features water elements, discovery zones, shaded groves, art pavilions, active circuits, and panoramic hills.
Surrounding the park, residents will also enjoy an elevated system of luxury signature amenities,12 Exclusive sporting clubs, and a network of grand indoor facilities, ensuring an unmatched lifestyle throughout the entire community. Chairman Muhammad Binghatti emphasized the significance of this moment, noting that both brands have returned “not only to build upon the success of their first collaboration, but to imagine what the future of living can become when automotive ingenuity and architectural innovation converge at a city scale.”
Mathias Geisen, member of the Board of Management, Mercedes-Benz Group AG, Sales and Customer Experience affirmed that “This masterplan represents the purest expression of the brand’s design philosophy, extending our DNA from the automobile into a living environment crafted with technical precision.”
Mercedes-Benz Places | Binghatti City, marks a new era, a world built beyond tomorrow, where mobility, architecture, technology and design function as one. This second collaboration between the two global leaders stands as a monumental step forward shaping not just a landmark development, but a blueprint for how the future of branded living in Dubai will be defined.
About Binghatti
Binghatti Holding Limited is one of the UAE’s fastest-growing real estate development companies, with a rapidly expanding portfolio that spans over 80 projects valued at more than AED 70 billion. Renowned for its pioneering branded residences, Binghatti has forged collaborations with global icons such as Bugatti, Mercedes-Benz, and Jacob & Co., creating architectural masterpieces that blend innovation with opulence. The company’s robust financial foundation and disciplined growth strategy are underpinned by its solid credit ratings, ‘BB-’ by Fitch and ‘Ba3’ by Moody’s, reflecting strong investor confidence and long-term stability. An architect by training, Chairman Muhammad Binghatti continues to shape the brand’s legacy of architectural excellence and uncompromising quality. Binghatti has delivered more than 12,000 residential units to date, with a portfolio spanning elegantly designed mainstream communities offering high-quality living at accessible prices to ultra-luxury residences that set new benchmarks in Dubai’s high-end real estate market. For more information, visit www.binghatti.com.
Media Contact
Yehia Mehdi
yehia.issa.dxb@gmail.com



Protera Acquires Redfig to Expand Enterprise IT Modernization Capabilities
Protera, a global leader in IT modernization and enterprise cloud services, today announced the acquisition of Redfig, an industry innovator in business transformation with a deep history in SAP application development, data integration, and intelligent automation. This acquisition expands Protera's service capabilities and accelerates its mission to deliver superior business outcomes through innovation, automation, and transformation.
"Redfig's deep experience in SAP BTP and application management services perfectly complements our mission to help enterprises modernize and innovate in the cloud," said Michael BeDell, CEO of Protera. "Together, we're expanding our portfolio to provide a suite of AI automation services that are unmatched in the industry and bring AI-driven, data-integrated capabilities to our clients to redefine what's possible in enterprise transformation."
With the addition of Redfig, Protera will offer customers a broad range of solutions, from enterprise AI automation, custom SAP applications and intelligent workflows to end-to-end data platform integrations. The acquisition also provides a foundation for expanding Protera's capabilities around BDC (Business Data Cloud), Clean Core initiatives and additional functional capabilities. The combined strength of Protera's managed cloud services and Redfig's deep business transformation expertise enables customers to optimize operations, accelerate digital transformation, and achieve measurable business impact.
"Redfig and Protera share a commitment to helping customers achieve meaningful business outcomes," said Olavo C. Figueiredo, CEO of Redfig. "By bringing together our SAP BTP and AI capabilities with Protera's global cloud leadership, we can accelerate transformation and deliver greater value at scale."
Redfig was among the earliest pioneers in the SAP BTP ecosystem and is an SAP Build Partner, certified to design and deploy SAP applications. Two of Redfig's innovative solutions are already available on the SAP Store: Redfig Partnerportal and Redfig Partnerflow.
"With the rapid acceleration of AI, the ability to harness enterprise data is fast becoming a competitive differentiator. Protera's leadership in cloud and infrastructure, paired with Redfig's expertise in data, platform engineering, and AI gives customers a clear path to build, automate, and scale faster," said Matthew C. Reddy, CTO of Redfig. "Together, we're building the foundation for the next era of innovation."
About Protera
Founded in 1998, Protera is a globally SAP-certified, Microsoft Azure, Amazon Web Services (AWS) and Google Cloud Platform (GCP) cloud migration and enterprise managed service provider offering a full suite of modern cloud, modern ERP, and modern work solutions. Learn more at www.protera.com.
About Redfig
Founded in 2019, Redfig LLC is a technology innovation firm focused on helping enterprises advance their operations through cutting-edge solutions, data modernization, and AI-driven automation. Redfig specializes in designing and integrating enterprise-grade applications across SAP Business Technology Platform (BTP), SAP ERP, and other cloud-native platforms, enabling organizations to streamline processes, enhance decision-making, and accelerate digital transformation for customers worldwide.
Media Contact
Megan Sharkey
Vice President of Marketing
m.sharkey@protera.com



FMG Announces Executive Leadership Appointments to Accelerate Growth and Innovation
FMG, the industry leader in marketing automation software for financial advisors and advisory firms, today announced executive leadership appointments to support continued growth and platform innovation for its enterprise and direct clients across RIAs, broker-dealers, wirehouses, regional firms, and insurance organizations.
FMG co-founder Dave Christensen has been appointed CEO, and Michelle Feinstein has joined FMG as chief product and strategy officer, succeeding Dave in his previous role. Additionally, Mo Ayadi joins as chief information officer, leading the information technology and security teams.
Scott White will transition to FMG's board of directors after nearly a decade as CEO. As a board member, Scott will continue to provide strategic guidance to FMG, building on his tenure strengthening the firm's position as the platform of choice for advisors.
As a co-founder of the business, Dave has been the longtime leader of product and strategy at FMG, and has led the company's evolution into a modern marketing automation platform used by advisors, firms, and enterprises across wealth and insurance. As AI fundamentally changes how technology platforms are built and how value is delivered to customers, Dave brings deep product leadership, experience and an innovation-forward perspective to guide FMG's next stage of growth.
Prior to joining FMG, Michelle most recently served as general manager of global financial services solutions and strategy at Salesforce, where she led product strategy and go-to-market execution for wealth and asset management clients. Previously, she held senior leadership roles at BNY Mellon Pershing and Albridge. Her experience in enterprise product leadership and commercial execution will support FMG's focus on creating a scalable, compliant marketing solution that seamlessly helps advisors and agents drive organic growth.
Mo brings deep experience building and scaling enterprise business systems, data platforms, and operational reporting. Most notably, during his tenure at LPL Financial, he served as senior vice president of Financial Technology Products, helping build a new wealth management platform, advance data strategy, and lead advisor CRM strategy. His addition ensures close collaboration across business systems, data infrastructure, and operational insights, empowering teams with the tools and information they need to make faster, smarter decisions.
"Our clients count on us to help them grow, whether they are individual advisors and agents, multi-advisor firms and agencies, or large enterprises," said Mark Casady, executive chairman of FMG. "Scott has been instrumental in building FMG into the trusted platform it is today, and we're grateful for his continued contributions as he transitions to our board. Dave has played a central role in FMG's product and platform evolution, and he is the right leader to guide the company forward. Michelle brings deep enterprise product experience and a strong track record of helping complex financial services firms connect strategy and execution. Together, this leadership team, including expanded product, technology, and data leadership, positions FMG to accelerate innovation, and deliver more personalized, AI-enabled growth for advisors, agents and enterprise marketing teams."
"GTCR is proud to support FMG as it continues to expand its impact across the wealth and insurance markets under Dave's leadership," said Michael Hollander, managing director at GTCR. "We are excited to partner with Dave, whose vision for the platform and deep understanding of FMG's strategy position the company well for its next phase of innovation as it accelerates product development and expands capabilities for advisors and enterprise firms. We look forward to continuing to work with Scott on the board and believe his insight on the market will be invaluable."
About FMG
FMG is the leading all-in-one marketing platform for financial advisors, insurance professionals, and enterprises, empowering them to scale compliant, client-centric marketing that drives organic growth. Trusted by more than 80,000 advisors who collectively reach over 45 million investors, approximately one in four investors in the United States, FMG is consistently ranked No. 1 in market share and customer satisfaction in the T3 Software Survey Report and has been recognized by WealthManagement.com as Best Marketing Automation Platform. Through its intuitive, centralized platform, FMG enables users to seamlessly manage websites, email, social media, texting, events, blogs, videos, and more, all from one place. By helping firms stay ahead of evolving trends and implement marketing best practices, FMG continues to set the standard for digital marketing in the wealth management industry.
Media Contact
Susan Theder
Chief Marketing Officer
susan.theder@fmgsuite.com
Iman Gadzhi Expands Digital Holdings with Full Acquisition of Consulting.com
Entrepreneur and investor Iman Gadzhi has acquired full ownership of Consulting.com, further expanding his digital holdings as part of a broader investment strategy centered on education, platforms, and infrastructure within the online economy.
Consulting.com is a legacy education and advisory company in the digital consulting space. According to Gadzhi, the business has historically generated between $75 million and $80 million in customer revenue over its lifecycle. Through the acquisition, Gadzhi assumes ownership of the Consulting.com domain, its intellectual property, and its existing education assets.
Gadzhi has publicly stated that the acquisition includes the entirety of the company and that Consulting.com will be revived and integrated into his broader advisory and education initiatives. He has described the company as a long-term asset and emphasized the importance of owning established platforms with durable intellectual property rather than relying solely on individual product launches.
The Consulting.com acquisition builds on Gadzhi’s expanding portfolio of digital-first businesses. Following investments made in 2025, he is a co-owner of Whop, a digital product and payments platform serving creators and online businesses. Gadzhi has stated that Whop processes over $100 million in monthly transaction volume and that his involvement extends beyond a passive investment role.
In addition to Consulting.com and Whop, Gadzhi has disclosed ownership stakes in several other companies, including Lyra, Hears, and Hills. These businesses span software, digital services, and consumer-facing brands, reflecting a diversified approach to ownership across multiple segments of the digital economy.
Across public appearances and long-form discussions, Gadzhi has consistently outlined a shift toward long-term ownership and infrastructure-focused investments. He has spoken extensively about moving away from short-term revenue strategies and toward acquiring and building platforms designed to support education, payments, and online business operations at scale.
The acquisition of Consulting.com reflects this broader strategy, positioning the company as a central asset within Gadzhi’s broader investment portfolio. By consolidating ownership of established education platforms while maintaining strategic positions in payments and software infrastructure, Gadzhi continues to expand his footprint across the digital business ecosystem.
As online education and creator-led commerce continue to mature globally, Gadzhi’s portfolio activity highlights a sustained focus on ownership, intellectual property, and platforms that enable long-term participation in the digital economy.
About Educate
Educate was founded by Iman Gadzhi, a British entrepreneur and digital marketer in 2018 and has been responsible for the education and careers of hundreds of thousands of students across the world. Initially specialising in agency growth and personal branding, Educate has expanded its curriculum to support careers in a wide variety of disciplines including eCommerce, copywriting and more. In 2022, the organisation adopted Educate as its official name, reflecting its mission statement and ethos. In 2023, Educate launched Digital Launchpad, the world’s subscription first e-learning portal dedicated to helping entrepreneurs start their career. To learn more, visit educate.io.
About Iman Gadzhi
Iman Gadzhi is an entrepreneur and investor with ownership interests across digital education, payments infrastructure, and online platforms. He is the owner of Consulting.com and a co-owner of Whop, alongside additional strategic investments in businesses operating across software, education, and consumer markets.
Media Contact
Hassan Al Rawi
hassan@educate.io



United Real Estate Launches First-of-Its-Kind Residential Real Estate Investment Program for Its Agents to Build Wealth as Investor-Entrepreneurs
United Real Estate today announced the launch of the United Residential Investment (URESI) Program, a comprehensive offering that empowers its agents to build long-term wealth and financial stability by becoming residential real estate investor-entrepreneurs.
Developed by United exclusively for its agents, URESI is a proprietary and comprehensive platform that guides agents through the process from being curious about investing in residential real estate to fully leveraging their real estate expertise to build income-producing and value-appreciating portfolios of properties.
The program rests on an interactive and supportive community led by deeply experienced residential real estate investor-experts and includes mentorship, 14 new world-class interactive courses, underwriting models, live deal-making events, workshops, on-demand resources, lending, insurance, rehab partners and more to ensure all URESI community members achieve their investment and wealth-building goals.
"Our agents now have a community of like-minded investment-curious agents, experienced agent investor professionals and the tools to create their own income and wealth-producing portfolios," said Dan Duffy, CEO of United Real Estate Group. "I can't think of a single new program launch that I have been more excited about in my thirty years as CEO of global companies than URESI. This is an exciting moment for United's agents."
Duffy added, "United exists for one and only one purpose: to improve the financial trajectory of our agents' and brokers' careers and lives. We don't believe any other national firm has ever offered a program like this to its agents. It's a one-of-a-kind program that presents a massive investment by United and a massive opportunity for our agents to benefit from property appreciation, cash flow and increased income. Real estate agents are uniquely positioned to succeed because of their insider market knowledge, ability to secure properties before other investors and, when trained, the ability to recognize high-yield rental opportunities. We are thrilled to bring the URESI community to our existing agents and will provide the program to new agents as they join one of our affiliated brokerages across the country."
A New Path to Building Wealth
According to the U.S. Bureau of Labor Statistics, the median annual wage for real estate agents is just over $58,000, with the top performers earning substantially more than the median. However, agents who invest in residential properties can significantly boost their cash flow and earnings with passive rental income while building wealth as their properties appreciate and their equity in those properties grows. The average 10-year ROI for residential real estate is 41.7% according to one report by iPropertyManagement. For a portfolio worth $4 million — 10 properties at $400K each — this could result in $1.6 million in appreciation over 10 years, plus $400K–$600K in mortgage principal paydown, depending on financing.
Mentorship and Community
Beyond financial gain, URESI has already become a community of motivated investors. Participants gain access to mentorship, deal flow and peer support — all within a built-in accountability ecosystem. The program is designed to help agents not only succeed but sustain their success and build generational wealth for their families and communities. From first-time investor agents to deeply experienced investor pros, URESI provides personal, professional and financial growth opportunities.
URESI aligns with United's core values of Family, Excellence, Fiscal Responsibility and Seeing Things Differently. It is part of United's broader Financial Wellness Program, which includes offerings like healthcare plans for independent contractors, retirement and wealth management services, estate & trust planning, investment vehicles and maximized agent compensation plans with the highest payout to agents in the industry, so agents take home almost everything they earn.
About United Real Estate
United Real Estate (United) — a division of United Real Estate Group — was founded with the purpose of offering solutions to real estate brokers and agents in the rapidly changing real estate brokerage industry. United provides the latest training, marketing and technology tools to agents and brokers under a flat-fee, transaction-based agent commission model. By leveraging the company's proprietary cloud-based Bullseye™ Agent & Broker Productivity Platform, United delivers a more profitable outcome for agents and brokers. United Real Estate operates in 35 states with 170 offices and more than 22,000 agents. The company produced over 73,000 transactions and $26.3 billion in sales volume in 2024. For more information, visit www.unitedrealestate.com or our official newsroom and access the full press release, multimedia assets and our latest news at the official United Newsroom.
About United Real Estate Group
United Real Estate Group (UREG) operates United Real Estate and United Country Real Estate, addressing the unique market needs of suburban, major metropolitan, urban and rural markets. Utilizing the cloud-based Bullseye™ Agent & Broker Productivity Platform, UREG offers the latest training, marketing and technology tools, producing a significant competitive advantage. The platform realizes over a decade-long investment in virtual agent and brokerage technology services and is powered by a 2.6 million listings data warehouse, generating over 3 million monthly visitors and 30,000+ leads per year. Together, the United Real Estate Group supports more than 600 offices and 25,000 real estate and auction professionals across four continents. United Real Estate Group produced 90,000 transactions and $30.7 billion in sales volume in 2024. Through its in-house advertising agency, UREG offers differentiating marketing support and collateral for specialized lifestyle property websites as well as access to a 800,000+ opt-in buyer database.
For more information about United Real Estate or United Country Real Estate, please visit UnitedRealEstate.com or UnitedCountry.com. To learn more about United Real Estate, brokerage succession planning, brokerage valuation and sale or franchising opportunities, visit GrowWithUnited.com. Agents interested in learning about career opportunities with United Real Estate can visit JoinUnitedRealEstate.com.
Media Contact
April Gonzalez
Media & Investor Relations
AGonzalez@UnitedRealEstate.com
+1 504-237-3500



CloudSEK Receives Strategic Investment from Connecticut Innovations to Drive Job Creation and Cybersecurity Research in the U.S.
CloudSEK, a predictive cyber threat intelligence firm specializing in AI-powered attack detection, today announced it has secured a strategic investment from Connecticut Innovations (CI), the strategic venture capital arm of the state of Connecticut, as part of its $10 million Series B2 round.
With this investment, CloudSEK becomes the first Indian-origin cybersecurity company to receive funding from a U.S. state-backed venture fund.
CloudSEK had previously raised $19 million in its Series B1 round and has now concluded the Series B round with this B2 tranche, comprising a mix of primary and secondary capital.
Building Jobs, Talent, and Cybersecurity Research in the U.S.
“Becoming the first Indian-origin cybersecurity company to receive backing from a U.S. state fund is a milestone for CloudSEK, as well as for the entire Indian cybersecurity ecosystem,” said Rahul Sasi, CloudSEK co-founder and CEO. “With Connecticut as our U.S. anchor, we are committed to creating jobs, investing in localized research, and strengthening cyber resilience in the Western world. CloudSEK is proud to advance its identity as a truly Indo-American cybersecurity company.”
Founded in 2015 by Sasi, a cybersecurity researcher-turned-entrepreneur, CloudSEK has evolved from a research-first initiative into one of the world’s most trusted cyber threat intelligence platforms, serving more than 300 enterprises across the BFSI, healthcare, technology, and government sectors.
The groundbreaking partnership with CI was forged after CloudSEK emerged as a top startup at VentureClash, CI’s global investment pitch competition. At the event, Sasi and product owner, Nivya Ravi, pitched a distinguished panel of judges, including Indra Nooyi, former Chair and CEO of PepsiCo; Connecticut Governor, Ned Lamont; and senior leaders from Endiya Partners, IITM Research Park, and Capria Ventures. Governor Lamont’s participation underscored the state’s commitment to attracting cutting-edge technology companies.
“At our 2025 VentureClash India pitch event, CloudSEK distinguished itself as a truly innovative provider of cybersecurity and predictive threat capabilities used by hundreds of businesses around the world,” said Alison Malloy, managing director, Investments at Connecticut Innovations. “We are excited to welcome CloudSEK to the CI portfolio and look forward to supporting their team as they establish their U.S. hub and pursue new customer and partnership opportunities in Connecticut.”
A Strategic U.S. Hub for an Indo-American Cybersecurity Powerhouse
CloudSEK will leverage this investment to accelerate its expansion in the U.S., with plans to establish its regional hub for operations, talent, and partnerships in Connecticut. The company is preparing to onboard strategic local talent and build deep collaborations with corporate partners, universities, and research ecosystems across the state.
CI funding will play an active role in enabling CloudSEK to:
- Recruit high-quality cybersecurity and AI talent from the region
- Establish partnerships with local academic and research institutions
- Build its U.S. regional headquarters in Connecticut
- Drive region-specific cybersecurity research and innovation
A Strong Foundation: Backed by Global Investors and Trusted by More Than 300 Enterprises
Prior to this round, CloudSEK’s Series B1 was led by U.S.-based strategic investor Commvault, with participation from MassMutual Ventures, Inflexor Ventures, Prana Ventures, and Tenacity Ventures. Early investors, including Meeran Family (Eastern Group), StartupXSeed, Neon Fund, and Exfinity Ventures continue to support the company’s long-term growth.
Additionally, CloudSEK recently announced a strategic partnership with Seed Group, a company of The Private Office of Sheikh Saeed bin Ahmed Al Maktoum, to deliver predictive cyber intelligence and AI-attack detection capabilities organizations across the UAE.
A New Chapter in Indo-American Cybersecurity Collaboration
This landmark investment not only amplifies CloudSEK’s global trajectory but also symbolizes the rise of Indian cybersecurity innovation on the world stage. By establishing strong roots in Connecticut and continuing to scale globally, CloudSEK is poised to strengthen cyber resilience across continents and redefine cross-border technology collaboration.
About CloudSEK
CloudSEK is a predictive cyber threat intelligence platform purpose-built to detect and anticipate AI-driven attack chains before they form. Our cloud-native SaaS platform continuously maps digital footprints, correlates weak signals, and identifies emerging attack paths to help organizations stay ahead of evolving threats. To learn more about how CloudSEK can strengthen your external security posture and deliver value from day one, visit www.cloudsek.com or drop a note to info@cloudsek.com.
About Connecticut Innovations
Connecticut Innovations (CI) is Connecticut's strategic venture capital arm and is the leading source of financing and ongoing support for innovative, growing companies. By offering equity and debt investments, strategic guidance and introductions to valuable partners, we help promising businesses thrive. For more information, please visit ctinnovations.com.
Media Contact
Shashank Shekhar
Manging Editor, CloudSEK
shashank.shekhar@cloudsek.com



Memphis Tours Strengthens Egypt’s Global Tourism Appeal with Thoughtfully Designed Travel Experiences
Memphis Tours is an old tourism company located in Giza, but still an essential factor that promotes Egypt as a global destination of significance. Through the incorporation of the local in-depth understanding and the promise to the coziness of the traveller and the authenticity of the culture, the business delivers the visitors with the journeys that benefit them discover Egypt in the expressive and well-organized style.
Egypt is a land of great history, diverse landscapes, and traditions that petition to the tourists. To Memphis Tours, the travel planning process assumes that knowledge, preparation, and one-on-one service are the pillars of great journeys. The philosophy has made the company a good dependable companion to the travelers who want a dependable and informative experience in their country excursions.
Experience Driven Travel Planning
Memphis Tours develops travel experiences that are not a typical sightseeing. Every itinerary is planned with much attention and knowledge of what tourists are interested in the most when touring Egypt. The company is equally emphasized on historical exploration, comfort, timing, and automatic coordination.
By having a group of skilled and technologically minded professionals on the ground, Memphis Tours would be guaranteeing the travelers to be guided accurately, be provided with efficient logistics as well as receptive and reliable assistance on their trip. This strategy will enable the visitor to specialize in exploration and leave the details of the same to the reliable professionals.
Exploring Egypt’s Heritage with Knowledgeable Guides
Memphis Tours can be recommended to those travelers who want to explore the country in a highly insightful and interactive way, as the tours of Egypt suggested by them would represent the most significant historical and cultural locations of Egypt. Visitors have an opportunity to visit what are recognized globally as great sights like the Pyramids of Giza, the ancient temples of Luxor, the old parts of Cairo and have an insightful experience of the past and the present in Egypt.
The knowledgeable guides recount the past to the present in the form of interesting explanations and local context through tours. These experiences are meant to be educational but at the same time easy to reach such that travelers of all classes can identify with the Egyptian heritage in a significant manner.
Memphis Tours has a range of services that include customized trips, family tours, and luxury tours, and all the customers of the company will be able to find a trip that fits their needs.
Discovering Egypt from the Nile
The Nile has influenced Egyptian civilization over a long period of time, and exploration of this legendary river provides one with a different view of the historical context of the state. The Memphis Tours presents refined Nile River cruises that give the visitor the prospect to visit and see the ancient sites at the tranquillity of coziness and peace.
The cruises generally move among Luxor and Aswan, and they have guided tours of the temples and monuments placed on the banks of the river. Visitors relish pre-planned tours, proficient advice and restful on-board activities that are both adventure-filled and comforting.
These trips present a perfect means to explore Egypt at a relaxed speed in addition to enjoying the cultural meaning of the Nile and the environs.
Personalized Service and Reliable Coordination
Another characteristic aspect of Memphis Tours is personalized service. Every itinerary is designed in consideration of the interest of the travelers, the style of a traveller and time considerations. The company involves clients in the process of making sure that all elements of the journey are satisfying to them.
Memphis Tours emphasize on reliability and quality in accommodation selection, transportation and guided activities. The company collaborates with reliable service providers to ensure that the comfort and safety of all the trips are of a similar standard.
Effective communication and preemptive planning are some of the factors that were used to make sure that the travelers are supported throughout the journey and even after the journey.
Encouraging Conscientious and Considerate Travel
Memphis Tours supports ethical travel that doesn't negatively impact Egypt's local communities or cultural heritage. By encouraging travelers to engage with local cuisine, customs, and way of life, the organization fosters deeper connections between travelers and destinations.
Serving International Travelers from Egypt
Memphis Tours is founded on Mourad St. in Giza and has an influential local base even though it caters to tourists worldwide. Visitors who intend to travel to Egypt have the company as their first choice because of its reliable services and qualified help.
As this orientation toward quality, professionalism and cultural richness, Memphis Tours will be committed to offering travel experiences that will encompass the real image of Egypt.
To know more about the available journeys and the option of creating a personalized travel plan, the travelers may access the webpage of Memphis Tours at their official website.
Company Information
- Company Name: Memphis Tours
- Address: Mourad St.
- City: Giza
- Country: Egypt
- Email: inquires@memphistours.com
- Website: www.memphistours.com
.jpeg)
.jpeg)

Tradomatix Emerges as Infrastructure Layer Connecting Hedge Fund Capital with Quantitative and AI Trading Intelligence
As global markets become increasingly driven by data, automation, and algorithmic decision-making, hedge funds are evolving beyond traditional trading desks and in-house development models. The modern trading environment now demands infrastructure that can integrate human expertise, quantitative models, and AI-driven systems at scale.
Tradomatix operates as a global trading technology platform designed to meet this demand. The platform enables hedge funds, quantitative traders, AI trading agents, brokers, and advanced market participants to deploy and operate trading systems within a unified, asset-class-agnostic environment. Tradomatix does not function as a broker, fund manager, or execution venue; instead, it provides the underlying infrastructure that supports strategy deployment, evaluation, and integration across global markets.
Re-Architecting How Hedge Funds Access Trading Intelligence
Hedge funds increasingly recognize that alpha generation is no longer confined to internal teams or single organizational structures. Quantitative insight, automation, and AI-driven intelligence are now distributed across independent traders, research specialists, and machine-built systems.
Tradomatix enables hedge funds to connect capital with this distributed intelligence through a shared technological framework. Quantitative traders and strategy builders deploy systems within institutional-grade infrastructure, while hedge funds allocate capital based on observed performance in live market conditions. This approach allows funds to access a broader spectrum of trading intelligence without restructuring internal teams or relinquishing control over capital and risk.
Unified Infrastructure for Hybrid Trading Operations
Trading operations today rarely rely on a single decision-making model. Human discretion, systematic strategies, and machine-driven execution increasingly coexist within the same portfolio. Tradomatix is built to support this hybrid structure.
The platform allows human-led strategies, quantitative models, and AI trading agents to operate simultaneously under standardized execution workflows. This enables hedge funds and trading organizations to monitor performance, assess correlations, and manage operational risk across diverse strategy types within one technological environment.
Operating on a non-custodial basis, Tradomatix ensures that institutions, traders, and brokers retain direct ownership and control over funds and execution while benefiting from shared infrastructure.
Enabling AI and Automation at Market Scale
AI-driven trading systems require reliable access to market data, execution pathways, and operational safeguards to function effectively in live environments. Tradomatix supports the deployment of AI trading agents and automated strategies through structured integration with real-market execution.
Machine-driven systems operate within defined parameters alongside human and quantitative workflows, reflecting the increasing convergence of discretionary, systematic, and AI-led trading approaches. The platform’s asset-class-agnostic design allows these systems to scale across markets without being limited by product-specific technology stacks.
Used Across Global Trading Ecosystems
Tradomatix is utilized by hedge funds, quantitative traders, AI trading agents, brokers, and advanced retail traders across global markets. This diverse participation reflects a broader industry trend toward shared infrastructure models that prioritize flexibility, interoperability, and performance visibility.
As automation, quantitative research, and artificial intelligence continue to shape global financial markets, infrastructure platforms that connect capital with specialized trading intelligence are becoming central to modern market operations. Tradomatix operates within this foundational layer.
About Tradomatix
Tradomatix is a global trading technology platform enabling hedge funds, quantitative traders, AI trading agents, autonomous bots, brokers, and advanced traders to integrate intelligent trading systems within a unified, non-custodial, asset-class-agnostic environment, supporting human, quantitative, and AI-driven trading strategies across global markets. For more information, visit www.tradomatix.com.
.jpeg)
.jpeg)

Releaf Launches First Legal Guidance Service for UK Medical Cannabis Patients
Releaf, the U.K.’s fastest-growing medical cannabis clinic, has today announced the launch of Releaf Protect, a first-of-its-kind for UK cannabis patients legal guidance service designed to strengthen patient confidence and protection.
Available to eligible members as part of Releaf+, the most comprehensive subscription plan for cannabis patients, Releaf Protect provides access to a dedicated legal helpline offering practical, situation-specific guidance for issues connected to lawful medical cannabis treatment in the U.K.
The launch follows the recent publication of an updated police report last week, which provided new guidance on how officers should approach interactions with legally prescribed medical cannabis patients. While this represents an important step forward for patient clarity, many patients still face uncertainty in high-pressure, real-world situations.
Tim Kirby, CEO of Releaf, said “Medical cannabis has been legal in the U.K. for several years, yet patients are still too often left carrying the burden of explaining the law in moments that can feel intimidating or stressful. As the U.K.’s leading medical cannabis healthcare provider, we believe our responsibility doesn’t end with prescribing, it extends to ensuring patients feel supported, protected and confident in everyday life. Releaf Protect is a meaningful step towards closing that gap, offering practical, responsible support at the moments it genuinely matters.”
The service is intended for specific, active situations, such as workplace disputes and interactions with police or other authorities. It forms part of Releaf’s wider commitment to leading innovation in the U.K. cannabis industry, alongside the Releaf Medical Cannabis Card, providing patients with clear, verifiable evidence of their prescription.
Kirby added, “Releaf continues to lead the industry in patient care and confidence. Our Medical Cannabis Card is uniquely supported by a secure two-factor verification process, enabling third parties to independently confirm a patient’s lawful prescription if it is ever challenged. The card and new legal guidance service, combined with our one of a kind technology platform, reflects our ongoing commitment to innovation, building real-world solutions that make medical cannabis safer, clearer and more accessible for patients across the U.K.”
The legal guidance is provided by Irwin Mitchell, a leading U.K. law firm with extensive experience advising on regulatory, employment and public law matters. Releaf does not receive the content of legal advice and does not influence the guidance given, ensuring patients receive independent, situation-specific support when it is needed most.
Together, these initiatives are designed to give prescribed medical cannabis patients greater confidence in everyday situations. By improving clarity, reducing misunderstandings and helping prevent unnecessary escalation, Releaf aims to promote better awareness and understanding of lawful medical cannabis prescriptions across the U.K.
About Releaf
Launched in 2024, Releaf is the U.K.'s fastest-growing and most-trusted medical cannabis clinic, serving patients through its advanced healthtech platform. With a prescriber base of over 50 specialists, we deliver evidence-based cannabinoid care directly to patients' homes through tailored treatment plans. Integrated with NHS systems, Releaf has transformed access to medicinal cannabis treatment in the U.K. and is now expanding internationally. For more information contact press@releaf.co.uk or visit releaf.co.uk.
Disclaimer
Medical cannabis laws and regulations vary by jurisdiction. Any information provided is for informational purposes only and does not constitute medical or legal advice. Patients should consult a licensed healthcare professional to determine whether medical cannabis is appropriate for their individual circumstances and should comply with all applicable local, state, and national laws governing its use. The availability, legality, and approved indications for medical cannabis may differ by location.
Media Contact
Sophie Stephen
Releaf
press@releaf.co.uk



Google Business Profile Reinstatement Services Launched in U.S. by Unsuspend Me and Search Scope
In response to a surge in suspended Google Business Profiles and Google's increasingly complex reinstatement system, Australian SEO agency Search Scope has partnered with U.S.-based brand Unsuspend Me to offer a fully managed Google Business Profile reinstatement service to companies across the United States.
This joint initiative connects Search Scope’s audit-based methodology and reinstatement expertise with Unsuspend Me’s U.S. operations, forming a two-tiered escalation system designed to recover suspended business profiles, including those that have failed multiple reinstatement attempts.
The service supports first-stage appeals, second appeals, and manual profile recovery processes, maintaining a documented 98% success rate across a wide range of reinstatement scenarios.
“Google’s process for reinstating suspended profiles has grown more unpredictable, requiring deep expertise rather than guesswork,” said Dorian Menard, founder of Search Scope. “Our framework gives U.S. businesses a legitimate way to reactivate Google Business Profiles, restore visibility, and reduce disruption to customer acquisition.”
Why Is Google Business Profile Suspension Reinstatement So Urgent for U.S. Businesses?
A suspended Google Business Profile removes the business from Google Search, Google Maps, and local pack results, immediately impacting visibility, calls, and lead flow. For many businesses, this translates directly into lost revenue, reduced customer engagement, and reputational risk.
When a profile is suspended, essential business information such as the telephone number, physical address, website URL, and trade name are no longer surfaced in local search results. This weakens the business’s digital footprint and creates friction in the customer journey.
Since December 2025, changes to Google’s reinstatement workflow have significantly reduced access to manual escalation. Many businesses are left navigating automated responses with limited feedback, increasing uncertainty and prolonging downtime.
“Businesses often don’t know why they were suspended or what to fix,” Menard said. “We focus on reversing that uncertainty through structured audits, evidence-based appeals, and clear communication.”
What is Google Business Profile Reinstatement?
Google Business Profile reinstatement is the process of restoring a suspended or disabled listing so it can once again appear in Google Search and Google Maps. Suspensions may result from policy violations, ownership conflicts, verification failures, content issues, or inconsistencies across a company’s website and supporting documentation.
Reinstatement typically requires submitting structured appeals supported by evidence such as business licenses, utility bills, address verification, and PDF documentation. Google’s systems evaluate the entire entity, meaning the website, content, citations, and digital footprint all influence the outcome.
Without a properly prepared appeal, many reinstatement attempts fail or stall indefinitely.
How Does the Search Scope and Unsuspend Me Reinstatement Process Work?
Search Scope and Unsuspend Me operate a structured, expert-led reinstatement workflow designed to address both straightforward and complex cases. The process includes:
- A full audit of the suspended profile, associated Google Account, website content, URL structure, and citation data to identify compliance issues
- Preparation and verification of documentation, including business licenses, utility records, address confirmation, telephone number validation, and PDF evidence files
- Manual escalation handling for profiles marked ineligible or repeatedly denied under standard appeal pathways
- Clear communication throughout the process via email updates and defined case workflows
- Post-reinstatement audits to reduce future suspension risk, including content cleanup, policy alignment, and ongoing profile hardening
All client documentation is handled with strict privacy and confidentiality standards.
“We built Unsuspend Me to fix broken business listings and get clients back online,” said Menard, who also serves as Director at Unsuspend Me. “Search Scope provides the strategic depth, while Unsuspend Me delivers focused execution for U.S. businesses.”
What Makes This Reinstatement Service Different from Other Agencies?
Most providers rely on generic appeal templates or basic form submissions that fail under Google’s current enforcement model. These approaches often lead to repeated denials or permanent suspension.
Search Scope and Unsuspend Me use a data-informed methodology based on hundreds of historical reinstatement cases. Each appeal is customized, audited, and aligned with current policy interpretation rather than automated guesswork.
The service has successfully reinstated profiles across high-risk and regulated categories, including legal services, medical practices, lead generation businesses, multi-location brands, and franchise networks.
“In many cases, clients reach us after being told reinstatement isn’t possible,” said Menard. “Our role is often to step in when other options have already failed.”
Who Benefits from These Google My Business Reinstatement Services?
The service supports a broad range of users, including small and medium-sized businesses, marketing agencies managing multiple listings, franchise operators, and international companies operating in the United States.
For these organizations, Google Business Profiles are a critical part of local visibility, trust, and customer acquisition. Reinstatement restores not just rankings, but continuity in marketing, advertising, and customer experience.
“If you can provide legitimate documentation and communicate clearly, we can help,” said Menard. “Suspensions are often procedural, not a reflection of business quality.”
What Are the Key Facts About This GBP Reinstatement Partnership?
- Google revised its reinstatement system in December 2025, limiting manual escalation and increasing automation.
- Search Scope and Unsuspend Me now offer a U.S.-focused reinstatement solution for suspended Google Business Profiles.
- Services include audits, appeals, escalations, and post-reinstatement risk mitigation.
- The partnership maintains a verified 98% success rate across complex and multi-appeal cases.
- Reinstatement services are available globally to English-speaking businesses.
- All cases are handled with strict attention to privacy, confidentiality, and regulatory compliance.
To learn more about Search Scope's Google Business Profile Reinstatement Service, visit https://searchscope.com.au/google-business-profile-reinstatement/.
About Search Scope
Search Scope is a Perth-based digital marketing and SEO agency specialising in measurable, lead-driven growth through search. The company helps businesses increase visibility, enquiries, and revenue by focusing on what actually moves the needle: organic search performance, Google Business Profile optimisation, local search dominance, and conversion-focused strategy. Search Scope works with local service providers, professional services, and established businesses that rely on inbound demand and want predictable outcomes rather than vanity rankings or generic reports. With a strong emphasis on technical accuracy, compliance with Google guidelines, and transparent performance tracking, Search Scope positions search as a scalable growth channel, not a marketing expense. The agency operates without long-term lock-in contracts and measures success by calls, leads, and booked enquiries, not superficial metrics. For more information, visit searchscope.com.au.
About Unsuspend Me
Unsuspend Me is a U.S.-based agency focused exclusively on Google Business Profile recovery. The company provides structured reinstatement support for suspended listings, handling documentation, appeals, escalation, and follow-up communication. For more information, visit unsuspendme.com or connect with Unsuspend Me on Linkedin or Facebook.
Media Contact
Dorian Menard
Director, Search Scope
seo@searchscope.com.au



Unlisted Expands Network of Real Estate Professionals with Suzanne O'Bryant of Landmark Sotheby’s International Realty
Unlisted, a groundbreaking digital real estate community focused on the 98% of homes that aren’t for sale — yet, announced today that Suzanne O'Bryant of Landmark Sotheby’s International Realty has joined the platform as the exclusive Local Expert for ZIP codes 28461, 28411, 28405, and 28409 across Southeastern North Carolina’s coastal communities.
Unlisted’s technology introduces new opportunities throughout the real estate landscape. Buyers gain access to homes that align with their lifestyle and long-term goals, not only those currently listed on the MLS. Homeowners receive greater clarity around how their property fits into local demand and can connect with interested buyers when the timing feels right. Real estate professionals benefit from tools that elevate their expertise, reinforce neighborhood presence, and encourage more meaningful conversations within their communities.
The platform leverages publicly available data to create a digital property profile for every home in the country. Buyers can search and organize these profiles into personalized collections, including homes that are not actively for sale. For homeowners, the profiles offer insight into market interest and reveal possibilities that may otherwise remain unexplored.
Each vetted real estate professional on Unlisted is assigned an agent profile that connects to every home profile within their designated ZIP codes. Only one agent is selected per ZIP code, underscoring Unlisted’s emphasis on trusted local representation, deep market knowledge, and credibility. As the network continues to grow, Unlisted remains focused on transparency, connection, and opportunity throughout the home journey.
Suzanne O'Bryant brings extensive coastal market expertise and a relationship-driven approach to her role as a Local Expert. With more than $350 million in career sales and consistent recognition as the top producing broker on Bald Head Island and in Brunswick County, Suzanne has built her reputation on open communication, strategic guidance, and a deep understanding of her clients’ lifestyles and goals.
Originally from Northern Virginia, Suzanne graduated with honors from NC State University with a degree in Communications and Public Relations. After a successful career in marketing and public relations, she and her family settled in Wilmington, where she transitioned into real estate full time. Her business quickly gained momentum, leading her to move to Bald Head Island full time in 2019. Deeply involved in the community, Suzanne serves on multiple boards and advisory groups and volunteers with the Bald Head Island Conservancy during the summer months.
Reflecting on the partnership, Suzanne shared her perspective: “Coastal North Carolina offers a way of life that is deeply personal and community driven. Unlisted gives buyers and homeowners a thoughtful way to explore opportunities earlier, and I’m excited to bring that insight to the coastal communities I know so well.”
Founder and CEO Katie Hill also commented on the announcement: “When we look for Local Experts, we look for agents who combine market leadership with genuine care for their communities. Suzanne’s experience, integrity, and commitment to her clients make her an outstanding addition to the Unlisted network.”
To learn more about Unlisted, visit UnlistedHomes.com. For Unlisted for Agents, visit UnlistedHomes.com/Agents.
To learn more about Suzanne O'Bryant, visit her Unlisted Profile or her website.
About Unlisted
Unlisted unlocks the potential in homes that aren’t for sale — yet. The company empowers home buyers to join the Waitlist for homes they love that aren’t for sale — in other words, unlisted. At the same time, homeowners collect a Waitlist of interested buyers for whenever the time comes to sell, giving everyone a head start. Unlisted also allows homeowners to control how their home is presented online; they can create a stunning up-to-date property profile that shows off the home’s best features and attracts more interest. With more time and more connection, Unlisted is a more human way to explore real estate that drives better outcomes for all. Selected for TechCrunch’s 2025 Startup Battlefield 200 as one of the top tech startups globally, and backed by HearstLab, Hearst Newspapers, VC414, StageNext Fund, and prominent angel investors, Unlisted gives buyers a competitive edge in today’s challenging housing market. For more information, visit UnlistedHomes.com.
Media Contact
Maura Racz
maura@unlistedinc.com



Arculus Integrates Yield.xyz to Bring Native and Liquid Staking to Its Cold Storage Wallet Platform
Arculus, the secure self-custody digital asset platform developed by CompoSecure (NYSE: CMPO), has integrated the Yield.xyz API to bring native and liquid staking directly into its cold storage wallet experience. The integration enables Arculus users to earn staking rewards across more than a dozen leading blockchain networks while maintaining full control of their private keys through Arculus’ hardware-backed security model.
With this release, Arculus expands beyond simple storage and transactions to become a full-featured self-custody staking platform. Users can now access onchain yield opportunities across major ecosystems including Ethereum, Solana, Tron, Avalanche, and Cosmos, all through a single, unified interface inside the Arculus Wallet App.
Secure Self-Custody Meets Native Staking
Arculus is a hardware-based cold storage wallet platform built around a metal smart card form factor that eliminates the need for batteries, charging cables, or USB dongles. The Arculus Key Card works in tandem with the Arculus Wallet App using a proprietary 3-factor authentication system that combines biometric authentication, a private 6-digit PIN, and the Arculus metal Key Card.
This layered security model allows users to maintain full self-custody of their digital assets while enabling intuitive “tap-to-transact” functionality for everyday onchain activity. With the integration of Yield.xyz, Arculus extends this security-first design to staking and yield generation.
Native and Liquid Staking Across Leading Networks
The Yield.xyz integration allows Arculus users to participate in staking across a wide range of proof-of-stake networks without leaving the wallet interface or relying on third-party platforms. Supported opportunities include:
- Ethereum staking via preferred validators and liquid staking through Lido and Rocket Pool
- Avalanche liquid staking via Benqi
- Tron liquid staking via JustLend
- Native staking for Celestia, Cosmos, Osmosis, Sei, Injective, Cardano, Polygon, Coreum, and additional networks
Yield.xyz acts as the staking infrastructure layer behind Arculus, providing standardized access to validators, liquid staking protocols, and yield products across chains. This allows Arculus to offer consistent staking flows across ecosystems while maintaining full self-custody for users.
Simplifying Onchain Participation
Rather than forcing users to manage different staking workflows for each blockchain, the Yield.xyz API abstracts protocol complexity and routes staking actions through a single integration. The result is a streamlined staking experience that feels native inside the Arculus wallet.
Users can discover staking opportunities, delegate assets, mint liquid staking tokens, and track rewards, all while their private keys remain secured by Arculus’ cold storage architecture.
“Yield.xyz gives Arculus users seamless access to staking across multiple ecosystems, all while maintaining the security expectations that hardware-based solutions require,” said Dr. Adam Lowe, chief product officer at Arculus. “Expanding staking support reflects our commitment to meeting user demand for broader onchain participation without compromising on self-custody.”
Infrastructure Built for Scale and Security
Yield.xyz is the unified yield infrastructure layer used by leading wallets, exchanges, and fintech platforms to power staking and DeFi products directly inside their applications. The API aggregates thousands of yield opportunities across 75-plus blockchains and provides production-grade access to validators, liquid staking protocols, money markets, and vaults through a single integration.
“Arculus provides an important access point for users who want secure, self-custodial staking,” said Serafin Lion Engel, CEO of Yield.xyz. “By integrating Yield.xyz, Arculus can offer staking across major ecosystems through a single connection while preserving the hardware-backed security model that their users trust.”
Expanding the Arculus Platform
Arculus is consistently ranked as one of the most innovative cold storage wallets in the market. In 2022, ABI Research named Arculus the Top Innovator in the cold storage wallet industry, citing its layered security model, metal smart card design, and ease of use.
Developed by CompoSecure, a global leader in secure authentication and payment technologies, Arculus brings institutional-grade security to everyday crypto users. The platform is designed for both new users entering crypto and experienced participants managing diversified onchain portfolios.
With staking now live, Arculus continues its evolution from a secure storage solution into a full-featured self-custody platform for earning, transacting, and participating across Web3.
About Yield.xyz
Yield.xyz is the leading unified yield infrastructure powering the next generation of fintech and Web3 finance. Through a single API, it enables wallets, neobanks, and fintech platforms to integrate onchain yield from staking, lending, and restaking protocols across more than 75 networks. Trusted by leaders including Ledger, Tangem, Zerion, Utila, and Deblock, Yield.xyz abstracts away blockchain complexity — delivering seamless, compliant, and revenue-driving yield experiences to millions of users worldwide. To learn more, visit yield.xyz.
Media Contact
Apurv Mishra
apurv@yield.xyz
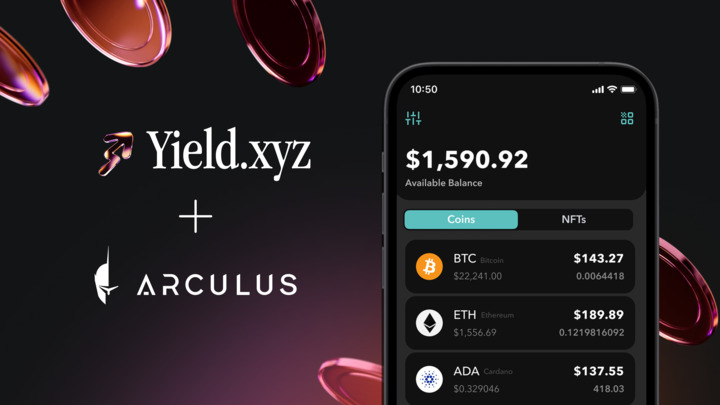


Stowers Institute Appoints Former Cerner Legal Chief as New General Counsel
The Stowers Institute for Medical Research has named Dan Devers as General Counsel, effective January 26, 2026. In this role, Devers will provide strategic and operational leadership on all legal matters across the Institute and its affiliates, including governance, compliance, intellectual property strategy, and partnerships that support the Institute's mission to uncover new knowledge in foundational biology and develop innovative ways to diagnose, treat, and prevent disease.
Devers joins the Stowers Institute as a seasoned legal and business executive with more than 20 years of experience. Most recently, he served as Executive Vice President and Chief Legal Officer at Cerner Corporation, the world's largest healthcare information technology company, before its acquisition by Oracle in 2022.
"Dan brings an extraordinary combination of legal depth, business acumen, and strategic vision," said Jonathan Thomas, CEO and Chairman of the Board for the Stowers Institute and Chairman, CEO and President of American Century Investments. "His leadership will be critical as we continue to build for the future, ensuring our governance structure and practices remain aligned with the scale, complexity and long-term strategy for the Stowers Institute, and advancing the long-term mission set forth by our founders, Jim and Virginia Stowers. I'm confident Dan will be a tremendous asset in supporting the breakthrough foundational research conducted by our scientists."
At Cerner, Devers negotiated the company's $28.3 billion sale to Oracle, which became one of the largest global transactions of 2022. In his role, he led global legal, regulatory, and risk management functions and developed an industry-leading portfolio of more than 650 patents.
"I am honored to join this remarkable organization during a dynamic time of scientific exploration and institutional growth," Devers said. "Legal strategy and governance play a vital role in enabling scientific excellence and collaboration. I look forward to advancing the Institute's extraordinary mission and ensuring our legal and business strategies maximize the global impact of the Institute's discoveries."
Each year, over 40% of American Century Investments' dividends are directed to the Stowers Institute to support its research. This has totaled more than $2 billion to date. Devers will ensure this unique and critical source of long-term funding is supported by legal strategies that sustain rigorous, mission-driven science.
"Strong science requires strong infrastructure," said Alejandro Sánchez Alvarado, Ph.D., President and Chief Scientific Officer of the Stowers Institute. "Dan's appointment signals our commitment to legal and organizational excellence as we pursue increasingly complex collaborations and tackle bold scientific questions that push the boundaries of what we know about life. His insight and leadership will be invaluable as we shape the future of foundational research."
Devers' appointment follows the retirement of Charles German, who has served as Executive Vice President and General Counsel for the Stowers Group of Companies since 2020. Devers will report directly to Jonathan Thomas.
Devers earned his J.D. with honors from the University of Missouri School of Law and a B.S. with honors in Mechanical Engineering from the University of Missouri. In addition to his corporate leadership roles, he has provided counsel and advisory support to academic institutions and nonprofit organizations, strengthening legal frameworks that support innovation and impact.
About Stowers Institute for Medical Research
Founded in 1994 through the generosity of Jim Stowers, founder of American Century Investments, and his wife, Virginia, the Stowers Institute for Medical Research is a non-profit, biomedical research organization with a focus on foundational research. Its mission is to expand our understanding of the secrets of life and improve life's quality through innovative approaches to the causes, treatment, and prevention of diseases.
The Institute consists of 20 independent research programs. Of the approximately 500 members, over 370 are scientific staff that include principal investigators, technology center directors, postdoctoral scientists, graduate students, and technical support staff. Learn more about the Institute at www.stowers.org and about its graduate program at www.stowers.org/gradschool.
Media Contact
Joe Chiodo
Director of Communications
press@stowers.org
+1 724-462-8529



USRA Appoints Dr. Brandon Thorne as Senior Vice President, Energy Programs
Universities Space Research Association (USRA) today announced the appointment of Dr. Brandon Thorne as Senior Vice President for Energy Programs, effective January 12, 2026. The appointment signifies USRA's strategic focus on anticipating and addressing the nation's evolving energy needs, particularly through the convergence of advanced energy systems, artificial intelligence, and quantum technologies.
For more than four decades, USRA has played a significant role in nuclear energy research, analysis, and workforce development, supporting federal missions and advancing scientific and technical excellence. The creation and appointment of this role positions USRA to build on that legacy while helping to shape the next generation of energy solutions during a period of renewed national and global investment in nuclear technologies.
"Energy is entering a transformative era, driven by advances in nuclear engineering, AI, quantum science, and the urgent need for resilient, secure, and clean power," said Dr. Elsayed Talaat, President and CEO, Universities Space Research Association. "The appointment of Brandon reflects our commitment to thinking strategically about what the nation needs next and ensuring that our research, partnerships, and talent are aligned to meet those needs."
Dr. Thorne brings over 20 years of experience in energy systems, nuclear science, policy, business development, partnership formation, aeronautical science, national security, and related fields with a demonstrated ability to lead complex, interdisciplinary initiatives across government, academia, and industry. Prior to joining USRA, he served as Senior Director, Office of the Vice President for the Princeton Plasma Physics Laboratory, where he supported the management and operations of a Department of Energy national laboratory. In that role, he acted as the second in command on a $1.6B prime contract, overseeing strategy, execution, and enterprise risk. He has held leadership positions in Nuclear Services and Technology and helped lead operations in the Nuclear and Particle Physics Directorate at Brookhaven National Laboratory. He also served in leadership roles at Lawrence Livermore National Laboratory and Savannah River Nuclear Solutions in Aiken, South Carolina.
Prior to his Department of Energy roles, Dr. Thorne led global operations supporting the Department of Defense, bringing expertise in aeronautical science and intelligence-informed systems integration, applying aeronautical science to advanced systems integration across energy and national security domains.
His leadership perspective is further shaped by advanced executive education at Harvard, Yale, Oxford, and Wharton, with a focus on strategy, governance, business, and enterprise leadership in complex institutions.
As Senior Vice President for Energy Programs, Dr. Thorne will lead USRA's energy strategy with a particular emphasis on nuclear energy innovation, AI-enabled energy systems, and the emerging role of quantum technologies in modeling, optimization, materials science, and national security applications. The role is designed to help propel USRA into a leadership position during what many describe as a new nuclear renaissance, marked by advanced reactors, digital engineering, and integrated energy-intelligence systems.
Dr. Thorne holds a Ph.D. from Southern University and Agricultural and Mechanical College, a master's in engineering from University of Alabama in Birmingham, an MBA from University of South Carolina, and a Bachelor of Science degree from Norfolk State University.
About USRA
Founded in 1969, the Universities Space Research Association (USRA) is an independent, nonprofit organization that advances space- and Earth-related science, engineering, and technology through innovative research, education, and workforce development programs. USRA partners with government agencies, academic institutions, and industry to address some of the nation's most complex scientific and technical challenges. For more information, visit www.usra.edu.
Media Contact
Suraiya Farukhi
sfarukhi@usra.edu
+1 443-812-6945



flynas Launches New Abha Operations Base in Cooperation with the Cluster2 Company and the Aseer Development Authority, Starting March 29
flynas, the leading low-cost airline in the world and the best low-cost carrier (LCC) in the Middle East, announced the establishment of a new operations base at Abha International Airport in cooperation with the leading airport operator, Cluster2 company, and the Aseer Development Authority, adding to its existing operations bases in Riyadh, Jeddah, Dammam, and Madinah, and becoming the first Saudi airline to operate flights from five different hubs across the Kingdom.
Beginning March 29, flynas will launch the first phase of this expansion, offering direct flights to 11 domestic and international destinations. The international network from Abha will include six permanent routes to Dubai, Cairo, Istanbul, and Addis Ababa, as well as seasonal service to Kuwait and Trabzon. These new routes complement the five domestic cities already served from Abha: Riyadh, Dammam, Jeddah, Madinah, and Tabuk. The leading LCC plans to gradually double the number of destinations linked to this new hub.
Bander Almohanna, CEO and managing director of flynas, said: “The launch of our new operations base in Abha represents a strategic investment designed to build an integrated national operating network. Beyond operational growth, the hub aims to stimulate economic and tourism development in the Aseer region, supporting the goal of transforming it into a year-round global tourist destination. This initiative directly aligns with the National Civil Aviation Strategy and the overarching objectives of Saudi Vision 2030.”
flynas, the leading low-cost airline in the world and the best LCC in the Middle East, and the first airline listed on the Saudi Exchange (Tadawul) operates 156 routes to more than 80 domestic and international destinations in 38 countries with more than 2000 weekly flights and has flown more than 80 million passengers since its launch in 2007, with the aim to reach 165 domestic and international destinations within its growth and expansion plan, and in line with the objectives of Vision 2030.
Passengers traveling with flynas can book their flights through all flynas booking channels: www.flynas.com, the flynas app, the call centre (9200 01234), or travel agents.
About flynas
flynas, the leading low-cost airline in world and the best in the Middle East, and the first airline listed on the Saudi Exchange (Tadawul), with a 66-aircraft fleet, operates more than 2000 weekly flights across 139 routes to more than 70 domestic and international destinations in 30 countries. Since its launch in 2007, flynas has transported more than 80 million passengers. Recently, in 2025, flynas was named the Best Low-Cost Airline in the Middle East for the eighth time in a row, winning in 2017, 2018, 2019, 2021, 2022, 2023, and 2024, and ranked among the top 10 low-cost airlines worldwide. As per the prestigious Skytrax, which is the most important global forum for the aviation industry. flynas has earned several accolades, including the Middle East's Leading Low-Cost Airline award from the World Travel Awards for eleven consecutive years (from 2015 to 2024), as well as being ranked in the 4-star low-cost carrier category, the highest category of the low-cost airline in the world by APEX rating. For more information, visit www.flynas.com.
Media Contact
flynas PR
flynas
woahmed@flynas.com
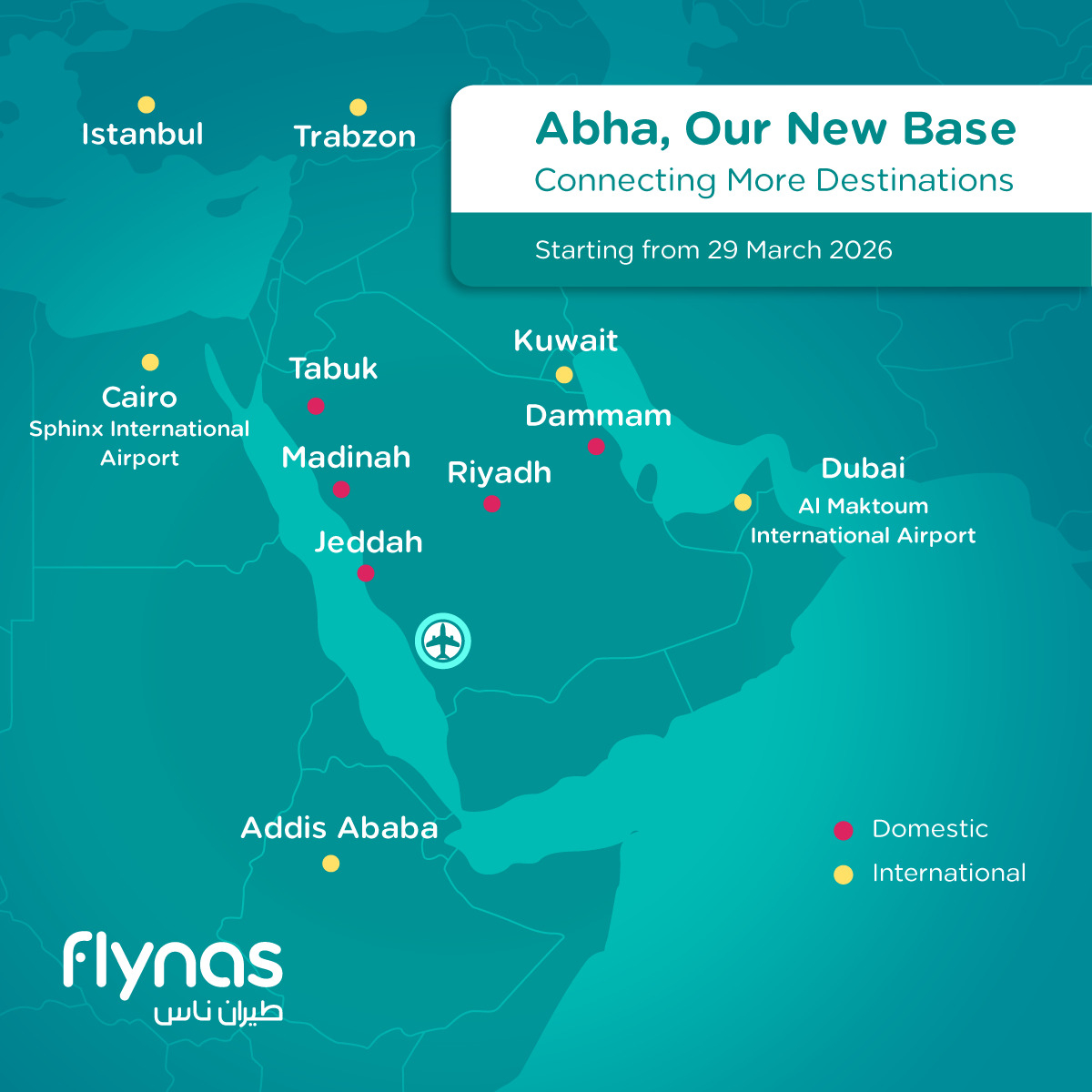



Turn your news into performance
EZ Newswire is the only news platform connecting the most influential organizations with the most trusted media outlets.